Forum Links
Related Threads
Coming Soon
Thread Information
Thread Actions
Thread Closed

New Thread

New Poll

Order
Teach to Learn
04-26-14 07:23 PM
 Singelli is Offline
| ID: 1013520 | 406 Words
Singelli is Offline
| ID: 1013520 | 406 Words
 Singelli is Offline
Singelli is Offline
| ID: 1013520 | 406 Words
Singelli
Level: 164





POSTS: 6820/8698
POST EXP: 1189395
LVL EXP: 56732525
CP: 67403.0
VIZ: 3154373

POSTS: 6820/8698
POST EXP: 1189395
LVL EXP: 56732525
CP: 67403.0
VIZ: 3154373

Likes: 3 Dislikes: 0
There is a substantial amount of evidence that shows writing leads to easier memorization. For example, if you need to study some vocabulary words for a vocabulary test, one of your best bets is to write the word and its meaning repetitively. There's something about doing this that imprints the information much more easily into your mind. Not only that, but another great way of learning is by.... believe it or not... teaching. I remember as a tutor, that I almost always felt like I gained knowledge and that my knowledge became stronger. When people ask you questions about a subject you've studied, you gain a deeper understanding by questioning yourself and reasoning through a logical answer. Most recently, I've been totally geeking out and searching out for opportunities to learn. I forgot what a great resource Khan Academy was, and as I listened to chemistry lectures, I was reminded of what successful teaching really looks and sounds like. I used to be totally confused by chemistry and feared it with a passion. I've had students come and ask me for help... and I'd promptly turn them away, telling them that it just wasn't my area. However, as I listened to Sal for a few hours, I became shocked by the realization that chemistry.... really wasn't so bad! I began taking notes and I have to admit my nerdiness here: I felt very giddy to be learning. XD Anyways, this joy made me want to create a chemistry thread here, but there were two problems with that: I only know what I've so far studied about chemistry. and I'd basically be restating the knowledge that Sal imparted to me through his Khan Academy videos. Anyways, thinking about all of this gave me an interesting idea... and I'd like to see if anyone would participate. Are you have trouble remembering what you've learned? Do you want to deepen your understanding of your class topics? If so.... try typing your notes here. Use them to the best of your ability, re-writing them as though you were teaching someone who knew absolutely nothing about the subject. Then, other people can read through your notes and correct areas of misunderstanding, or ask questions (providing a challenge for yourself). Besides, I think it'd be very interesting to see what everyone is learning, and if anyone posts here, I'll be learning something myself! I'll make an example post in a few moments. Most recently, I've been totally geeking out and searching out for opportunities to learn. I forgot what a great resource Khan Academy was, and as I listened to chemistry lectures, I was reminded of what successful teaching really looks and sounds like. I used to be totally confused by chemistry and feared it with a passion. I've had students come and ask me for help... and I'd promptly turn them away, telling them that it just wasn't my area. However, as I listened to Sal for a few hours, I became shocked by the realization that chemistry.... really wasn't so bad! I began taking notes and I have to admit my nerdiness here: I felt very giddy to be learning. XD Anyways, this joy made me want to create a chemistry thread here, but there were two problems with that: I only know what I've so far studied about chemistry. and I'd basically be restating the knowledge that Sal imparted to me through his Khan Academy videos. Anyways, thinking about all of this gave me an interesting idea... and I'd like to see if anyone would participate. Are you have trouble remembering what you've learned? Do you want to deepen your understanding of your class topics? If so.... try typing your notes here. Use them to the best of your ability, re-writing them as though you were teaching someone who knew absolutely nothing about the subject. Then, other people can read through your notes and correct areas of misunderstanding, or ask questions (providing a challenge for yourself). Besides, I think it'd be very interesting to see what everyone is learning, and if anyone posts here, I'll be learning something myself! I'll make an example post in a few moments. |
Vizzed Elite
Affected by 'Laziness Syndrome'
Registered: 08-09-12
Location: Alabama
Last Post: 3141 days
Last Active: 3116 days
| Singelli |
Affected by 'Laziness Syndrome'
Registered: 08-09-12
Location: Alabama
Last Post: 3141 days
Last Active: 3116 days
04-26-14 09:01 PM
 Singelli is Offline
| ID: 1013563 | 3910 Words
Singelli is Offline
| ID: 1013563 | 3910 Words
 Singelli is Offline
Singelli is Offline
| ID: 1013563 | 3910 Words
Singelli
Level: 164





POSTS: 6821/8698
POST EXP: 1189395
LVL EXP: 56732525
CP: 67403.0
VIZ: 3154373

POSTS: 6821/8698
POST EXP: 1189395
LVL EXP: 56732525
CP: 67403.0
VIZ: 3154373

Likes: 0 Dislikes: 0
Over the past couple of days, I've studied three different subjects: Chemistry, Spanish, and Programming.
The Chemistry and Programming I've been studying through Khan Academy. The Spanish I've been studying through a program called ProSpanish on youtube. Programming I haven't gotten very deep into the programming lessons, but so far, they aren't too complicated. Khan has it's own program with it's own 'commands', but I'm assuming that the skills used therein can be aptly used with various coding languages. That is, understanding their program will impart the skills needed for using programming languages. Thus, it's hard to say how I really feel about learning what I have so far. That being said, I can share some of the things I've learned relative to the program. Probably the most 'rounded' information I've acquired are a few programing vocabulary words: function - a command used to tell the program what to do. These are things we use here in the vizzed layout maker, like background: or border:. They're followed by a colon and whatever parameters are necessary. Functions are 'closed' by using a semi-colon. parameters - the details of any function used in the programming language. Depending on the command, these parameters could be colors, sizes, or locations. documentation - a list of functions used in a program and a detailed account of what each function does and how its parameters are set. Since different languages and different programs will have a wide variety of functions and parameters, it's advised not to try and memorize them all. Instead, you should be able to find documentation somewhere for functions less commonly used. For vizzed, this would be like the text on the left hand side of the posting box. The descr Although the program on Khan Academy is unique, I'm sure many coding languages are fairly similar... much in the way languages share common roots and certain words are recognizable across different languages. (For example, any English speaker would be able to recognize the word 'invitaci?n' despite not knowing the Spanish language.) For this reason, I'm going to share the commands that I've learned, along with their understood parameters: rect(#,#,#,#);- 'rect' is the command to draw a rectangle, it has four numerical parameters. The first number listed should be the horizontal location of the upper left corner of the rectangle. The second number is the vertical location of the upper left corner of the rectangle. The third number is how wide the rectangle should be, and the last number is how tall it should be. Although I'd have to look to be sure, I believe vizzed's layout maker uses the parameters in a different order. The way location is determined is the same however. Within the coding space, the upper left corner is always considered (0, 0). If you know anything about math, this means that the point of origination is here. If we were to suddenly exist within that space and stand still, we'd stand on the upper left hand corner. If we travel directly to the right, this would be our horizontal distance, which affects the first coordinate. All movement is measured in pixels. Therefore, if the ONLY movement I make is to walk 10 pixels to the right, my coordinates would be (10, 0). If I were to move downwards, this would be the vertical distance. If I do not move horizontally, but I travel down 30 pixels, my coordinates would then be (0, 30). Diagonal motion is only accomplished by a combination of horizontal and vertical motion. Thus, a coordinate of (20, 50) would mean that I have traveled 20 pixels to the right, and 50 pixels down from that upper left corner of the coding space. I've created an illustration as a demonstration: 
After determining the location of your upper left corner of your rectangle, you state its width and height, also measured in pixels. ellipse(#,#,#,#); - This command creates an ellipse much in the same manner that the rect function works. However, the first two numbers represent the location of the center of the ellipse, while the third number represents the width of the ellipse, and the fourth number is the height of the ellipse. Thus a command to draw a circle isn't necessary. if you set the height and width to be equal, a circle will be created. line(#,#,#,#);- This command creates a line. The first two numbers are coordinates of one endpoint of the line, while the next two numbers are the coordinates of the other endpoint. background(#,#,#); - This sets a background color for every object listed after the function. What it really does is draw a filled rectangle behind everything coded after it. It's important to understand the order of your coding in order to get it to work properly. If you use the background function, everything coded afterwards will appear on top of that filled rectangle. This means if you draw a circle in your coded area.... then use the background command after, the circle will be covered and no longer visible. The three numbers are a color (rgb). The first number is the amount of red, the second number is the amount of green, and the third number is the amount of blue. 0 represents none of that color being used, and 255 represents the max amount of that color possible. This means if you use (255, 0, 0), your background will be completely red. If you wanted the purplest color possible (totally just made up a word XD), you'd max out the red and blue by using (255, 0, 255). stroke(#,#,#); - This sets the colors of the lines for any item coded after this function is applied. The three numbers are an rgb number. noStroke(); - This is a command which actually requires no parameters. However, it still needs the parenthesis, and the semicolon signifying that the command is complete. This function tells the program to remove all lines for objects created once it's applied. fill(#,#,#); - This is akin to the bucket function in Microsoft's Paint program. It fills any object drawn after it is applied, and the three numbers are rgb colors. noFill(); - Again, this command has no parameters. It removes any color from the insides of objects drawn after it is applied. var ( As an example, Khan Academy encourages you to work on a mini-project after several lessons, and the purpose of the project is to fill a dinner plate with whatever items you wish. One of the things I chose to design into the plate were peas. I'd want all the peas to be the same size, but there would be a lot of peas on the plate! If I drew fifty peas on the plate, then decided I didn't like their size... I'd have to re-size each and every one of them individually. OR.... I could use the variable function. It might look something like this: var pea = 15; From then on, anywhere I type the word pea, the program will read that word as having the value of 15. I could have a code, then, that looks like this: var pea = 15; ellipse(30,30,pea, pea); ellipse (32, 28, pea, pea); ellipse (29, 31, pea, pea); etc etc. These three ellipses would be in different locations, but they'd all have a diameter of 15 pixels. Instead of changing each number if I wanted to change the size, I could change the very first line to something like var pea = 20. Every ellipse I've coded afterwards would then automatically change size, their diameters now being 20. In essence, it works exactly like a variable in algebra would. If I define x to be 3, then I write an equation like 3x2- 2x - 4, it'd be understood that every x should be ---- Although knowing the functions is important, it's equally important to understand the order of coding. You see, it DOES matter what order you code things in. If you code an ellipse, and then a square in the same location, the square will be drawn on TOP of the ellipse, rendering part of the ellipse invisible depending on the size of your square. Likewise, if you draw a square, and then place an ellipse over the square, then part of the square could be hidden by the ellipse. Due to the nature of coding, any function can be overridden if used more than once in the same program. As an example, let's take a look at my 'pea' example earlier. var pea = 15; fill(0,0,255); ellipse(30,30,pea, pea); ellipse (32, 28, pea, pea); ellipse (29, 31, pea, pea); Placing the fill function before the three ellipses means all three ellipses will be the same color. However, if I were to instead code: var pea = 15; fill(0,0,255); ellipse(30,30,pea, pea); fill(0,255,0); ellipse (32, 28, pea, pea); ellipse (29, 31, pea, pea); ... then the FIRST pea would be green. The next fill function, however, overrides what was written before, so the last two peas would be blue. Chemistry To my understanding, chemistry is a study of the basic building blocks of our world. If you were to observe every basic material in our world, you would discover a list of elements. Elements are things like carbon, gold, and hydrogen.... all located on the periodic table. They are the materials that everything on earth is made of. If you were to take these elements and break them down to their tiniest components... in other words, if you were to continually cut off pieces of the element (even at a microscopic level), you'd eventually discover atoms. The word 'atom' actually comes from some other word in another language which means 'uncuttable' or 'indivisible'. They are ridiculously small, and in fact, a million of them can fit into the cross section of a hair strand. Interestingly enough, as we study the anatomy of an atom, 99.99% of an atom is empty space. (Try wrapping your mind around that one...) An atom is made of three different parts: -Protons define an element on the periodic table. For example, EVERY helium atom will have the exact same number of protons, and every Lithium atom will have the same number of protons. The number of protons is equal to the atomic number on the periodic table. Since hydrogen is the first element, for example, all hydrogen atoms have one proton. Likewise, all Silicon atoms contain 14 protons. Protons are located in the nucleus (the center of the atom), and they have a positive charge. -Neutrons are also located in the nucleus of an atom, but they have a neutral charge. (They are not positively or negatively charged.) The number of neutrons -may- change, but atoms in their natural untouched state will have the same number of neutrons as protons. If there is an abnormal number of neutrons, the atom is called an isotope. For example, if a carbon atom (#12 on the periodic table) were to contain 14 neutrons instead of 12 neutrons , that carbon atom would be called an isotope. -Electrons orbit the nucleus of an atom and have a negative charge. Although there are normally an equal number of electrons as protons, electrons are what interact with other atoms, and thus vary once a reaction with another element takes place. Most people are familiar with the Bohr model of an atom (created by Neil Bohr around a hundred years ago), but the problem is that it's not entirely accurate. Thinking of an atom as being similar to this model can create problems with comprehension when studying electrons. In case you aren't certain of what I'm talking about, the Bohr model looks like this: 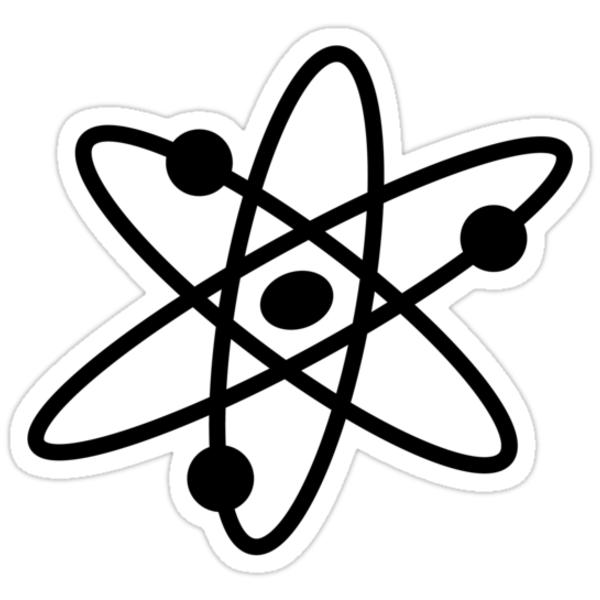
The splotch in the center is supposed to represent an atom's nucleus, while the lines and dots around it are supposed to be electrons orbiting the nucleus, much in the way planets orbit the sun. This is NOT the way electrons move, however. Their position, direction, and speed aren't perfectly predictable, nor are they guaranteed. Instead, electrons will be somewhere NEAR the nucleus... and their positions can only be guessed using probability. A more accurate model might look like this: 
or this: _and_4s_(color)_Electron_Clouds.jpg)
Although these both look extremely complex, they'll make more sense later. Basically, if you look at the first accurate image I showed, the shaded area represents a 'cloud' around the nucleus. An electron may be anywhere inside that cloud, but it has a higher probability of being in the areas which are darker and closer to the nucleus... and a smaller probability of being further out in the cloud where the shading is lighter. This area where the electron might be traveling is called an orbital. --- Another number that might show up on the periodic table is often found under its symbol. As an example, the number 12.0107 might appear under the Carbon symbol (element 6). This number represents the weight of a carbon atom. Now, obviously.... an atom's weight is not something easily measured. An atom is so ridiculously small that measurement of their 'weight' requires a completely new unit of measurement. Since all protons and neutrons weigh the same amount, they are used to define the new standard of measure. One proton or neutron weighs 1 atomic mass unit, or 1 AMU. If this is the case, however, it would make sense for Carbon to weigh 12 AMU... not 12.0107 AMU. The reason it's not 12 is because the number on the periodic table represents the average weight of any carbon atom anywhere on earth. Remember that an atom might not have the 'normal' amount of neutrons. One carbon isotope has 8 neutrons instead of six... so it would weigh 14 AMU. 12 AMU and 14 AMU would average to 13 AMU, but there isn't an equal number of untouched carbon atoms and carbon isotopes. Carbon with 8 neutrons is a rare find. If you were to average ALL of the carbon isotopes with ALL of the untouched carbon atoms, the average would be 12.0107. One way to write the symbol for carbon is like such: 
The 6 represents the number of protons each carbon atoms has, while the number 12 represents the combined amount of protons and neutrons. Carbon's isotope with 8 neutrons would thus be written as such: 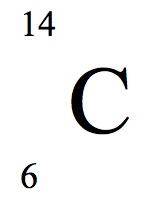
If you know your periodic table well enough, the '6' on these symbols is unnecessary, so you'll sometimes see them without the bottom left number. Electrons don't get figured into the weight at all. Their weight is infinitessimally small, and it takes more than 1800 electrons to match the weight of one proton or neutron. Thus, their weight is negligible. --- Of course, in chemistry, we're interested in observing the reactions between elements and chemicals and whatnot. These reactions, however, occur thanks to the energy around an atom and how that energy can be manipulated. Basically, the further away an electron is from the nucleus of the atom, the more energy it has. (Imagine a wrecking ball swinging on a short chain, and one swinging on a much longer chain. The one swinging on the larger chain would have much more potential of creating damage because there is a lot more potential energy in its swing.) Since all electrons have a negative charge, they are attracted to the positive protons in the nucleus, but repelled by each other in the orbitals. The orbitals thus have levels or 'shells' in which so many electrons are usually found. Remember, the location of an electron isn't a guarantee. It's simply a probability. So the orbital shell that is closest to the nucleus will most likely contain 2 electrons, and is the first 'shell' to be filled before the electrons get sick of each other and feel the need to orbit further away. Of course, like any 'magnetic attraction', the further away the electrons are from that positive charge in the center of the atom... the weaker the attraction is. The electrons further out might have more energy, but they're also easier to 'pluck away' and more likely to leave their atom for another that has a stronger attraction. The attraction between an electron and the nucleus is called the Colomb force. (I have no idea if I spelled that correctly.) --- A few other smaller notes of importance, in order to understand the periodic table: * Rows are called periods * Columns are called groups * 1st group on periodic chart = alkaline metals * 2nd group = alkaline earth metals * Groups 3-12 = transitional metals * Group 17 = halogens * Group 18 = noble gases --- Since the outermost electrons on an atom are the ones most likely to react, it's important to understand just how many are there and where they are located. For this reason, there is a notation used to represent the configurations of electrons. As an example, the notation for hydrogen would be 1s1 and the notation for helium would be 1s2. The large number in front of the 's' is the period in which the element is located. Since hydrogen and helium are in the first row, there is a one in the front. ?The periodic table is arranged so that each period represents the next shell, or level of orbital, that the electrons are likely to be found in. As an example, any element found in the fourth row of the periodic table has four 'shells' in which the electrons are likely to be found, with the outermost electrons in the fourth shell. The superscr Lithium has three electrons. However, the first shell only has space for two electrons so the third electron moves into a second shell further away from the nucleus. (Lithium is in the second period, so it has two shells). Thus, the configuration would be written like this: 1s22s1. This means that there are 2 electrons in the first shell, and 1 electron in the second shell. Once more electrons start being added to an atom however, there are more ways in which to be repelled by each other. For this reason, funny things start happening. Two electrons can whiz about close to the nucleus. Once more atoms are added, they need to spin about a bit further away, in the second shell. Two in the second shell might be alright whizzing around in a circle, but a third electron (third in the second shell, fifth in the entire atom) begins to feel crowded. It tries to find a route which keeps it furthest away from the other electrons in its shell. I have a difficult time describing it, so let me share a drawing I wrote down in my notebook. (Fair warning: I'm a terrible artist. And I have terrible penmanship.) 
The three axes represent a three dimensional space. The pencil dots show the possible places one of the electrons is likely to travel in (upwards and downwards in a dumbbell like shape), whereas the pen dots show the possible locations of yet another electron (side to side in a dumbbell shape). If yet ANOTHER electron were to be added, it'd be likely to travel in a dumbbell shaped area that was facing from front to back. You can kind of see what I mean by the second picture I posted under the electron discussion. A more complete list of these funny paths looks like this: 
I really like this image because it even gives you a good idea of WHERE on the periodic table certain paths take place. A 3-d representation of the dumbbell orbitals looks like this: 
These dumbbell orbitals are given a different title so that people studying chemistry know what the atom looks like. The 's' orbitals are rather spherical, and the dumbbell orbitals are called 'p' orbitals. Let's look at the element carbon, for example. Carbon is element number 6 and thus has 6 electrons naturally. The first two will stay within the first shell, closest to the nucleus, so we'd write 1s2. Once that shell is filled, the next electrons will move in the second shell. Two of them will orbit in a spherical space... the s subshell. It's still a part of the second shell.... but only two will likely whiz about in that area. This means that so far, we have 1s22s2. There are still two more electrons flying about, but they are repelled by the two already in the second shell. Therefore, they start flying in a dumbbell shape.... the p orbitals. Up to six electrons can travel the p orbitals, but we only have 2 electrons left, so the configuration is 1s22s22p2. This reads as follows: There are two electrons in the first shell, which is spherical. There are four electrons in the second shell, but two of them are in the s orbital, and 2 of them are in the p orbitals. If you've noticed, there are other shapes in that first linked image that's shaped like the periodic table. That's because once there are four shells in an atom, there are more 'sub-shells' an electron can travel. Groups 3-12 on the periodic table offer a new travel path for electrons, and these are called 'd' orbitals. Likewise, the rows pulled out of a periodic table (usually shown at the bottom) have new shapes called f orbitals. It gets a bit trickier when more electrons are added to an atom, so let's first try working out a few more electron configurations. First, let's try Silicon. Silicon has 14 electrons. The first two will fit into the first shell. Then, two more will fit into the second shell before they must move into the dumbbell shape. Remember....the dumbbell shape IS a part of the second shell... it's just a differently shaped orbital. Six electrons can fly around in the p orbitals. Thus, so far the configuration would be 1s22s22p6. However, this only accounts for ten electrons. There are still four more which need to be placed. Those four, however, will be too repelled by the eight in the second shell. They will move to a shell even further out: the third shell. The third shell, like all others, will fit 2 electrons in a spherical orbit. Thus, we can add onto the configuration: 1s22s22p63s2. There are 2 electrons left, which will still be in the third shell, but will fly in the dumbbell shape. The electron configuration for silicon is1s22s22p63s23p2 (I'm still working on this) The Chemistry and Programming I've been studying through Khan Academy. The Spanish I've been studying through a program called ProSpanish on youtube. Programming I haven't gotten very deep into the programming lessons, but so far, they aren't too complicated. Khan has it's own program with it's own 'commands', but I'm assuming that the skills used therein can be aptly used with various coding languages. That is, understanding their program will impart the skills needed for using programming languages. Thus, it's hard to say how I really feel about learning what I have so far. That being said, I can share some of the things I've learned relative to the program. Probably the most 'rounded' information I've acquired are a few programing vocabulary words: function - a command used to tell the program what to do. These are things we use here in the vizzed layout maker, like background: or border:. They're followed by a colon and whatever parameters are necessary. Functions are 'closed' by using a semi-colon. parameters - the details of any function used in the programming language. Depending on the command, these parameters could be colors, sizes, or locations. documentation - a list of functions used in a program and a detailed account of what each function does and how its parameters are set. Since different languages and different programs will have a wide variety of functions and parameters, it's advised not to try and memorize them all. Instead, you should be able to find documentation somewhere for functions less commonly used. For vizzed, this would be like the text on the left hand side of the posting box. The descr Although the program on Khan Academy is unique, I'm sure many coding languages are fairly similar... much in the way languages share common roots and certain words are recognizable across different languages. (For example, any English speaker would be able to recognize the word 'invitaci?n' despite not knowing the Spanish language.) For this reason, I'm going to share the commands that I've learned, along with their understood parameters: rect(#,#,#,#);- 'rect' is the command to draw a rectangle, it has four numerical parameters. The first number listed should be the horizontal location of the upper left corner of the rectangle. The second number is the vertical location of the upper left corner of the rectangle. The third number is how wide the rectangle should be, and the last number is how tall it should be. Although I'd have to look to be sure, I believe vizzed's layout maker uses the parameters in a different order. The way location is determined is the same however. Within the coding space, the upper left corner is always considered (0, 0). If you know anything about math, this means that the point of origination is here. If we were to suddenly exist within that space and stand still, we'd stand on the upper left hand corner. If we travel directly to the right, this would be our horizontal distance, which affects the first coordinate. All movement is measured in pixels. Therefore, if the ONLY movement I make is to walk 10 pixels to the right, my coordinates would be (10, 0). If I were to move downwards, this would be the vertical distance. If I do not move horizontally, but I travel down 30 pixels, my coordinates would then be (0, 30). Diagonal motion is only accomplished by a combination of horizontal and vertical motion. Thus, a coordinate of (20, 50) would mean that I have traveled 20 pixels to the right, and 50 pixels down from that upper left corner of the coding space. I've created an illustration as a demonstration: 
After determining the location of your upper left corner of your rectangle, you state its width and height, also measured in pixels. ellipse(#,#,#,#); - This command creates an ellipse much in the same manner that the rect function works. However, the first two numbers represent the location of the center of the ellipse, while the third number represents the width of the ellipse, and the fourth number is the height of the ellipse. Thus a command to draw a circle isn't necessary. if you set the height and width to be equal, a circle will be created. line(#,#,#,#);- This command creates a line. The first two numbers are coordinates of one endpoint of the line, while the next two numbers are the coordinates of the other endpoint. background(#,#,#); - This sets a background color for every object listed after the function. What it really does is draw a filled rectangle behind everything coded after it. It's important to understand the order of your coding in order to get it to work properly. If you use the background function, everything coded afterwards will appear on top of that filled rectangle. This means if you draw a circle in your coded area.... then use the background command after, the circle will be covered and no longer visible. The three numbers are a color (rgb). The first number is the amount of red, the second number is the amount of green, and the third number is the amount of blue. 0 represents none of that color being used, and 255 represents the max amount of that color possible. This means if you use (255, 0, 0), your background will be completely red. If you wanted the purplest color possible (totally just made up a word XD), you'd max out the red and blue by using (255, 0, 255). stroke(#,#,#); - This sets the colors of the lines for any item coded after this function is applied. The three numbers are an rgb number. noStroke(); - This is a command which actually requires no parameters. However, it still needs the parenthesis, and the semicolon signifying that the command is complete. This function tells the program to remove all lines for objects created once it's applied. fill(#,#,#); - This is akin to the bucket function in Microsoft's Paint program. It fills any object drawn after it is applied, and the three numbers are rgb colors. noFill(); - Again, this command has no parameters. It removes any color from the insides of objects drawn after it is applied. var ( As an example, Khan Academy encourages you to work on a mini-project after several lessons, and the purpose of the project is to fill a dinner plate with whatever items you wish. One of the things I chose to design into the plate were peas. I'd want all the peas to be the same size, but there would be a lot of peas on the plate! If I drew fifty peas on the plate, then decided I didn't like their size... I'd have to re-size each and every one of them individually. OR.... I could use the variable function. It might look something like this: var pea = 15; From then on, anywhere I type the word pea, the program will read that word as having the value of 15. I could have a code, then, that looks like this: var pea = 15; ellipse(30,30,pea, pea); ellipse (32, 28, pea, pea); ellipse (29, 31, pea, pea); etc etc. These three ellipses would be in different locations, but they'd all have a diameter of 15 pixels. Instead of changing each number if I wanted to change the size, I could change the very first line to something like var pea = 20. Every ellipse I've coded afterwards would then automatically change size, their diameters now being 20. In essence, it works exactly like a variable in algebra would. If I define x to be 3, then I write an equation like 3x2- 2x - 4, it'd be understood that every x should be ---- Although knowing the functions is important, it's equally important to understand the order of coding. You see, it DOES matter what order you code things in. If you code an ellipse, and then a square in the same location, the square will be drawn on TOP of the ellipse, rendering part of the ellipse invisible depending on the size of your square. Likewise, if you draw a square, and then place an ellipse over the square, then part of the square could be hidden by the ellipse. Due to the nature of coding, any function can be overridden if used more than once in the same program. As an example, let's take a look at my 'pea' example earlier. var pea = 15; fill(0,0,255); ellipse(30,30,pea, pea); ellipse (32, 28, pea, pea); ellipse (29, 31, pea, pea); Placing the fill function before the three ellipses means all three ellipses will be the same color. However, if I were to instead code: var pea = 15; fill(0,0,255); ellipse(30,30,pea, pea); fill(0,255,0); ellipse (32, 28, pea, pea); ellipse (29, 31, pea, pea); ... then the FIRST pea would be green. The next fill function, however, overrides what was written before, so the last two peas would be blue. Chemistry To my understanding, chemistry is a study of the basic building blocks of our world. If you were to observe every basic material in our world, you would discover a list of elements. Elements are things like carbon, gold, and hydrogen.... all located on the periodic table. They are the materials that everything on earth is made of. If you were to take these elements and break them down to their tiniest components... in other words, if you were to continually cut off pieces of the element (even at a microscopic level), you'd eventually discover atoms. The word 'atom' actually comes from some other word in another language which means 'uncuttable' or 'indivisible'. They are ridiculously small, and in fact, a million of them can fit into the cross section of a hair strand. Interestingly enough, as we study the anatomy of an atom, 99.99% of an atom is empty space. (Try wrapping your mind around that one...) An atom is made of three different parts: -Protons define an element on the periodic table. For example, EVERY helium atom will have the exact same number of protons, and every Lithium atom will have the same number of protons. The number of protons is equal to the atomic number on the periodic table. Since hydrogen is the first element, for example, all hydrogen atoms have one proton. Likewise, all Silicon atoms contain 14 protons. Protons are located in the nucleus (the center of the atom), and they have a positive charge. -Neutrons are also located in the nucleus of an atom, but they have a neutral charge. (They are not positively or negatively charged.) The number of neutrons -may- change, but atoms in their natural untouched state will have the same number of neutrons as protons. If there is an abnormal number of neutrons, the atom is called an isotope. For example, if a carbon atom (#12 on the periodic table) were to contain 14 neutrons instead of 12 neutrons , that carbon atom would be called an isotope. -Electrons orbit the nucleus of an atom and have a negative charge. Although there are normally an equal number of electrons as protons, electrons are what interact with other atoms, and thus vary once a reaction with another element takes place. Most people are familiar with the Bohr model of an atom (created by Neil Bohr around a hundred years ago), but the problem is that it's not entirely accurate. Thinking of an atom as being similar to this model can create problems with comprehension when studying electrons. In case you aren't certain of what I'm talking about, the Bohr model looks like this: 
The splotch in the center is supposed to represent an atom's nucleus, while the lines and dots around it are supposed to be electrons orbiting the nucleus, much in the way planets orbit the sun. This is NOT the way electrons move, however. Their position, direction, and speed aren't perfectly predictable, nor are they guaranteed. Instead, electrons will be somewhere NEAR the nucleus... and their positions can only be guessed using probability. A more accurate model might look like this: 
or this: _and_4s_(color)_Electron_Clouds.jpg)
Although these both look extremely complex, they'll make more sense later. Basically, if you look at the first accurate image I showed, the shaded area represents a 'cloud' around the nucleus. An electron may be anywhere inside that cloud, but it has a higher probability of being in the areas which are darker and closer to the nucleus... and a smaller probability of being further out in the cloud where the shading is lighter. This area where the electron might be traveling is called an orbital. --- Another number that might show up on the periodic table is often found under its symbol. As an example, the number 12.0107 might appear under the Carbon symbol (element 6). This number represents the weight of a carbon atom. Now, obviously.... an atom's weight is not something easily measured. An atom is so ridiculously small that measurement of their 'weight' requires a completely new unit of measurement. Since all protons and neutrons weigh the same amount, they are used to define the new standard of measure. One proton or neutron weighs 1 atomic mass unit, or 1 AMU. If this is the case, however, it would make sense for Carbon to weigh 12 AMU... not 12.0107 AMU. The reason it's not 12 is because the number on the periodic table represents the average weight of any carbon atom anywhere on earth. Remember that an atom might not have the 'normal' amount of neutrons. One carbon isotope has 8 neutrons instead of six... so it would weigh 14 AMU. 12 AMU and 14 AMU would average to 13 AMU, but there isn't an equal number of untouched carbon atoms and carbon isotopes. Carbon with 8 neutrons is a rare find. If you were to average ALL of the carbon isotopes with ALL of the untouched carbon atoms, the average would be 12.0107. One way to write the symbol for carbon is like such: 
The 6 represents the number of protons each carbon atoms has, while the number 12 represents the combined amount of protons and neutrons. Carbon's isotope with 8 neutrons would thus be written as such: 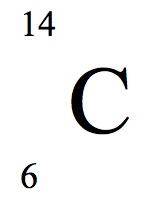
If you know your periodic table well enough, the '6' on these symbols is unnecessary, so you'll sometimes see them without the bottom left number. Electrons don't get figured into the weight at all. Their weight is infinitessimally small, and it takes more than 1800 electrons to match the weight of one proton or neutron. Thus, their weight is negligible. --- Of course, in chemistry, we're interested in observing the reactions between elements and chemicals and whatnot. These reactions, however, occur thanks to the energy around an atom and how that energy can be manipulated. Basically, the further away an electron is from the nucleus of the atom, the more energy it has. (Imagine a wrecking ball swinging on a short chain, and one swinging on a much longer chain. The one swinging on the larger chain would have much more potential of creating damage because there is a lot more potential energy in its swing.) Since all electrons have a negative charge, they are attracted to the positive protons in the nucleus, but repelled by each other in the orbitals. The orbitals thus have levels or 'shells' in which so many electrons are usually found. Remember, the location of an electron isn't a guarantee. It's simply a probability. So the orbital shell that is closest to the nucleus will most likely contain 2 electrons, and is the first 'shell' to be filled before the electrons get sick of each other and feel the need to orbit further away. Of course, like any 'magnetic attraction', the further away the electrons are from that positive charge in the center of the atom... the weaker the attraction is. The electrons further out might have more energy, but they're also easier to 'pluck away' and more likely to leave their atom for another that has a stronger attraction. The attraction between an electron and the nucleus is called the Colomb force. (I have no idea if I spelled that correctly.) --- A few other smaller notes of importance, in order to understand the periodic table: * Rows are called periods * Columns are called groups * 1st group on periodic chart = alkaline metals * 2nd group = alkaline earth metals * Groups 3-12 = transitional metals * Group 17 = halogens * Group 18 = noble gases --- Since the outermost electrons on an atom are the ones most likely to react, it's important to understand just how many are there and where they are located. For this reason, there is a notation used to represent the configurations of electrons. As an example, the notation for hydrogen would be 1s1 and the notation for helium would be 1s2. The large number in front of the 's' is the period in which the element is located. Since hydrogen and helium are in the first row, there is a one in the front. ?The periodic table is arranged so that each period represents the next shell, or level of orbital, that the electrons are likely to be found in. As an example, any element found in the fourth row of the periodic table has four 'shells' in which the electrons are likely to be found, with the outermost electrons in the fourth shell. The superscr Lithium has three electrons. However, the first shell only has space for two electrons so the third electron moves into a second shell further away from the nucleus. (Lithium is in the second period, so it has two shells). Thus, the configuration would be written like this: 1s22s1. This means that there are 2 electrons in the first shell, and 1 electron in the second shell. Once more electrons start being added to an atom however, there are more ways in which to be repelled by each other. For this reason, funny things start happening. Two electrons can whiz about close to the nucleus. Once more atoms are added, they need to spin about a bit further away, in the second shell. Two in the second shell might be alright whizzing around in a circle, but a third electron (third in the second shell, fifth in the entire atom) begins to feel crowded. It tries to find a route which keeps it furthest away from the other electrons in its shell. I have a difficult time describing it, so let me share a drawing I wrote down in my notebook. (Fair warning: I'm a terrible artist. And I have terrible penmanship.) 
The three axes represent a three dimensional space. The pencil dots show the possible places one of the electrons is likely to travel in (upwards and downwards in a dumbbell like shape), whereas the pen dots show the possible locations of yet another electron (side to side in a dumbbell shape). If yet ANOTHER electron were to be added, it'd be likely to travel in a dumbbell shaped area that was facing from front to back. You can kind of see what I mean by the second picture I posted under the electron discussion. A more complete list of these funny paths looks like this: 
I really like this image because it even gives you a good idea of WHERE on the periodic table certain paths take place. A 3-d representation of the dumbbell orbitals looks like this: 
These dumbbell orbitals are given a different title so that people studying chemistry know what the atom looks like. The 's' orbitals are rather spherical, and the dumbbell orbitals are called 'p' orbitals. Let's look at the element carbon, for example. Carbon is element number 6 and thus has 6 electrons naturally. The first two will stay within the first shell, closest to the nucleus, so we'd write 1s2. Once that shell is filled, the next electrons will move in the second shell. Two of them will orbit in a spherical space... the s subshell. It's still a part of the second shell.... but only two will likely whiz about in that area. This means that so far, we have 1s22s2. There are still two more electrons flying about, but they are repelled by the two already in the second shell. Therefore, they start flying in a dumbbell shape.... the p orbitals. Up to six electrons can travel the p orbitals, but we only have 2 electrons left, so the configuration is 1s22s22p2. This reads as follows: There are two electrons in the first shell, which is spherical. There are four electrons in the second shell, but two of them are in the s orbital, and 2 of them are in the p orbitals. If you've noticed, there are other shapes in that first linked image that's shaped like the periodic table. That's because once there are four shells in an atom, there are more 'sub-shells' an electron can travel. Groups 3-12 on the periodic table offer a new travel path for electrons, and these are called 'd' orbitals. Likewise, the rows pulled out of a periodic table (usually shown at the bottom) have new shapes called f orbitals. It gets a bit trickier when more electrons are added to an atom, so let's first try working out a few more electron configurations. First, let's try Silicon. Silicon has 14 electrons. The first two will fit into the first shell. Then, two more will fit into the second shell before they must move into the dumbbell shape. Remember....the dumbbell shape IS a part of the second shell... it's just a differently shaped orbital. Six electrons can fly around in the p orbitals. Thus, so far the configuration would be 1s22s22p6. However, this only accounts for ten electrons. There are still four more which need to be placed. Those four, however, will be too repelled by the eight in the second shell. They will move to a shell even further out: the third shell. The third shell, like all others, will fit 2 electrons in a spherical orbit. Thus, we can add onto the configuration: 1s22s22p63s2. There are 2 electrons left, which will still be in the third shell, but will fly in the dumbbell shape. The electron configuration for silicon is1s22s22p63s23p2 (I'm still working on this) |
Vizzed Elite
Affected by 'Laziness Syndrome'
Registered: 08-09-12
Location: Alabama
Last Post: 3141 days
Last Active: 3116 days
| Singelli |
Affected by 'Laziness Syndrome'
Registered: 08-09-12
Location: Alabama
Last Post: 3141 days
Last Active: 3116 days
(edited by Singelli on 04-27-14 12:14 AM)
04-27-14 07:36 AM
 Juliet is Offline
| ID: 1013858 | 300 Words
Juliet is Offline
| ID: 1013858 | 300 Words
 Juliet is Offline
Juliet is Offline
| ID: 1013858 | 300 Words
Juliet
Level: 151





POSTS: 5806/6750
POST EXP: 348455
LVL EXP: 43237456
CP: 10738.8
VIZ: 1380871

POSTS: 5806/6750
POST EXP: 348455
LVL EXP: 43237456
CP: 10738.8
VIZ: 1380871

Likes: 1 Dislikes: 0
(Here goes a pretty out-of-topic post! Just so others won't be too confused specially in the first part of the programming article, here are some things that I'd like to point out: 1. Functions aren't commands. They "contain" the commands. With that said, you can still give commands even without using functions, depending on the programming language used. C or C++ would always use the "int main()" function and won't be manipulated properly without it. However, languages like 'Assembly' don't even have the function feature and instead implement the jump (goto or JMP) feature which is a tedious way of doing things. 2. Parameters aren't always the details inside the function. As I've said, function is a container and it's not always present. Parameters can exist even without any functions typically together with global variables when declaring (they exist outside the main codes but could be called anytime and anywhere unlike the "Formal Parameters"). Formal parameters (with local variables) are what you use inside the functions and could only be used "specifically" inside its parent function. 3. Documentation... it's fine calling it that but you'll have more luck searching using the word 'reference' instead... I'm assuming you are studying wolfram? (Because you said it's Khan and I don't know any other Khan's xD.) If yes, that is awesome! I also like the idea for this thread but I'm afraid I'd have no time to type math equations. However, I might be able to do this if I could just upload pictures of our lectures and problems solved in class and textbooks (and resolved by me) with minimal typing instead. It's easier to write than type when it comes to math (at least for me). Just so others won't be too confused specially in the first part of the programming article, here are some things that I'd like to point out: 1. Functions aren't commands. They "contain" the commands. With that said, you can still give commands even without using functions, depending on the programming language used. C or C++ would always use the "int main()" function and won't be manipulated properly without it. However, languages like 'Assembly' don't even have the function feature and instead implement the jump (goto or JMP) feature which is a tedious way of doing things. 2. Parameters aren't always the details inside the function. As I've said, function is a container and it's not always present. Parameters can exist even without any functions typically together with global variables when declaring (they exist outside the main codes but could be called anytime and anywhere unlike the "Formal Parameters"). Formal parameters (with local variables) are what you use inside the functions and could only be used "specifically" inside its parent function. 3. Documentation... it's fine calling it that but you'll have more luck searching using the word 'reference' instead... I'm assuming you are studying wolfram? (Because you said it's Khan and I don't know any other Khan's xD.) If yes, that is awesome! I also like the idea for this thread but I'm afraid I'd have no time to type math equations. However, I might be able to do this if I could just upload pictures of our lectures and problems solved in class and textbooks (and resolved by me) with minimal typing instead. It's easier to write than type when it comes to math (at least for me). |
Vizzed Elite
Affected by 'Laziness Syndrome'
Registered: 05-10-09
Location: Manila, PH (Asia)
Last Post: 2185 days
Last Active: 191 days
3rd Place in the July 2009 VCS Competition! |
Affected by 'Laziness Syndrome'
Registered: 05-10-09
Location: Manila, PH (Asia)
Last Post: 2185 days
Last Active: 191 days
Post Rating: 1 Liked By: Singelli,
04-27-14 08:44 AM
 Singelli is Offline
| ID: 1013920 | 270 Words
Singelli is Offline
| ID: 1013920 | 270 Words
 Singelli is Offline
Singelli is Offline
| ID: 1013920 | 270 Words
Singelli
Level: 164





POSTS: 6822/8698
POST EXP: 1189395
LVL EXP: 56732525
CP: 67403.0
VIZ: 3154373

POSTS: 6822/8698
POST EXP: 1189395
LVL EXP: 56732525
CP: 67403.0
VIZ: 3154373

Likes: 0 Dislikes: 0
Juliet : Don't apologize! This is EXACTLY my intent for this thread, and your post isn't off topic at all! The only way for me to perfect my learning (and this is how I want the thread to be utilized), is by showing YOU what I've 'learned'. Once you read through and see where I'm not understanding, you can help me fix those wrong ideas. That being said, I do have to say your points totally flew over my head! XD I mean.. I get that I used / defined the vocabulary the wrong way, but other than your correction on the word 'documentation', that totally left me at a loss. I know I defined the words incorrectly, but I only have a vague idea on why. lol It's because I don't know anything about the languages or terms like "implement the jump". As for wolfram... do you mean wolframalpha.com? (LOVE that site, btw!) I'm not, if that's what you mean. I'm using a wonderful site called khanacademy.org, which is a learning center for hundreds of topics. The videos in my opinion are done very, very well. Many lessons even have activities complete with a large random problem base, hints, and solutions. And you can definitely post pictures of your notes. ^.^ I don't think it can be AS beneficial though, because you're not 're-telling' it and getting your most complete thoughts down. However, it can certainly still help. Go for it! I'd love to see what and how you're learning. I know other countries learn things in different ways, too, which would make it even more interesting to see. That being said, I do have to say your points totally flew over my head! XD I mean.. I get that I used / defined the vocabulary the wrong way, but other than your correction on the word 'documentation', that totally left me at a loss. I know I defined the words incorrectly, but I only have a vague idea on why. lol It's because I don't know anything about the languages or terms like "implement the jump". As for wolfram... do you mean wolframalpha.com? (LOVE that site, btw!) I'm not, if that's what you mean. I'm using a wonderful site called khanacademy.org, which is a learning center for hundreds of topics. The videos in my opinion are done very, very well. Many lessons even have activities complete with a large random problem base, hints, and solutions. And you can definitely post pictures of your notes. ^.^ I don't think it can be AS beneficial though, because you're not 're-telling' it and getting your most complete thoughts down. However, it can certainly still help. Go for it! I'd love to see what and how you're learning. I know other countries learn things in different ways, too, which would make it even more interesting to see. |
Vizzed Elite
Affected by 'Laziness Syndrome'
Registered: 08-09-12
Location: Alabama
Last Post: 3141 days
Last Active: 3116 days
| Singelli |
Affected by 'Laziness Syndrome'
Registered: 08-09-12
Location: Alabama
Last Post: 3141 days
Last Active: 3116 days
04-27-14 02:02 PM
 Juliet is Offline
| ID: 1014060 | 276 Words
Juliet is Offline
| ID: 1014060 | 276 Words
 Juliet is Offline
Juliet is Offline
| ID: 1014060 | 276 Words
Juliet
Level: 151





POSTS: 5807/6750
POST EXP: 348455
LVL EXP: 43237456
CP: 10738.8
VIZ: 1380871

POSTS: 5807/6750
POST EXP: 348455
LVL EXP: 43237456
CP: 10738.8
VIZ: 1380871

Likes: 0 Dislikes: 0
... I went way too technical with the terms didn't I? 1. What I mean with this would be... functions are used in programming to contain the commands, thus, they are not commands. Functions are usually used to call a specific command when needed. 2. Parameters are the declarations and when you are giving values to variables(var). Say if there's something like var x, or var y = 4, then that's a parameter, x or y are the variables, and 4 is the argument. However, if it is something like printf("hello world"); (A classic example to demonstrate how to tell the program to show something on the screen. This will print the words "hello world"), then it is a command. Parameters can exist outside functions, and some functions can exist without any formal parameters, therefore, parameters aren't always the details inside the function. 3. Documentation is more of a term used for collection of files or programs, so it's better to use reference instead if you're searching about program functionality and how to manipulate it. Hahaha I love wolframalpha as well! I'm more of referring to the wolfram language itself though... -> https://www.youtube.com/watch?v=_P9HqHVPeik it's a new programming language and it seems really cool. And I've always believed Khan and wolfram are related but it seems like I'd need to do more research. 1. What I mean with this would be... functions are used in programming to contain the commands, thus, they are not commands. Functions are usually used to call a specific command when needed. 2. Parameters are the declarations and when you are giving values to variables(var). Say if there's something like var x, or var y = 4, then that's a parameter, x or y are the variables, and 4 is the argument. However, if it is something like printf("hello world"); (A classic example to demonstrate how to tell the program to show something on the screen. This will print the words "hello world"), then it is a command. Parameters can exist outside functions, and some functions can exist without any formal parameters, therefore, parameters aren't always the details inside the function. 3. Documentation is more of a term used for collection of files or programs, so it's better to use reference instead if you're searching about program functionality and how to manipulate it. Hahaha I love wolframalpha as well! I'm more of referring to the wolfram language itself though... -> https://www.youtube.com/watch?v=_P9HqHVPeik it's a new programming language and it seems really cool. And I've always believed Khan and wolfram are related but it seems like I'd need to do more research. |
Vizzed Elite
Affected by 'Laziness Syndrome'
Registered: 05-10-09
Location: Manila, PH (Asia)
Last Post: 2185 days
Last Active: 191 days
3rd Place in the July 2009 VCS Competition! |
Affected by 'Laziness Syndrome'
Registered: 05-10-09
Location: Manila, PH (Asia)
Last Post: 2185 days
Last Active: 191 days
(edited by Juliet on 04-27-14 02:04 PM)
04-28-14 05:54 PM
 Singelli is Offline
| ID: 1014566 | 141 Words
Singelli is Offline
| ID: 1014566 | 141 Words
 Singelli is Offline
Singelli is Offline
| ID: 1014566 | 141 Words
Singelli
Level: 164





POSTS: 6824/8698
POST EXP: 1189395
LVL EXP: 56732525
CP: 67403.0
VIZ: 3154373

POSTS: 6824/8698
POST EXP: 1189395
LVL EXP: 56732525
CP: 67403.0
VIZ: 3154373

Likes: 0 Dislikes: 0
Juliet : Hm. Well, it's still a bit over my head, but perhaps as I continue studying, I will become better versed in the language. XD As for the wolfram language, I watched the video you offered, and it seems to be the very idea/language behind wolframalpa. I didn't watch the video for very long because it was far more complicated than I could follow. However, it seemed to work like wolframalpha itself works. Now there's an impressive program if I ever did see one! I can not get enough of that site! I'm pretty sure though, that Khan Academy is not related to wolfram. Khan is mainly teacher created video lessons and exercises. I never finished my chemistry notes or posted my Spanish notes, so I might do that once I get back from shopping, by editing it into this post. I'm pretty sure though, that Khan Academy is not related to wolfram. Khan is mainly teacher created video lessons and exercises. I never finished my chemistry notes or posted my Spanish notes, so I might do that once I get back from shopping, by editing it into this post. |
Vizzed Elite
Affected by 'Laziness Syndrome'
Registered: 08-09-12
Location: Alabama
Last Post: 3141 days
Last Active: 3116 days
| Singelli |
Affected by 'Laziness Syndrome'
Registered: 08-09-12
Location: Alabama
Last Post: 3141 days
Last Active: 3116 days
Links
Page Comments
This page has no comments


 User Notice
User Notice 